Discovery
Capabilities Spanning Discovery to Clinical Development
From early discovery to clinical development, we integrate advanced computation, peptide engineering, and translational expertise to accelerate the path from concept to therapy.
Leveraging ProteinStudio™, we are advancing a pipeline of both internal and partnered drug discovery projects. Our goal is clear: to discover and develop novel medicines that will significantly improve patient outcomes.
Overview
A Robust Pipeline of Novel Therapeutics Addressing High Unmet Medical Need
SORT1
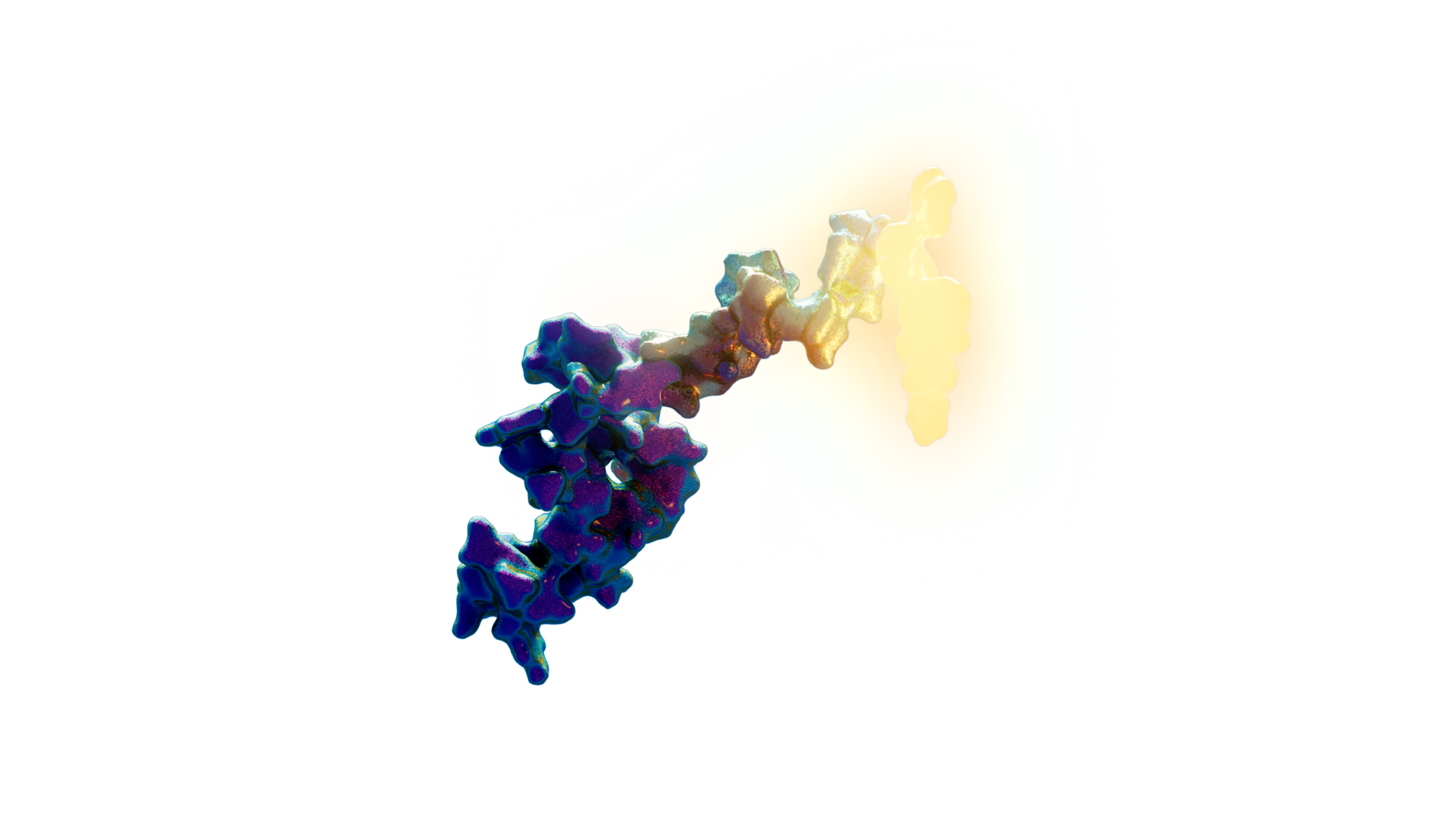
Sortilin [SORT1] Delivery System
We have computationally designed and optimized high-affinity peptides to target the cell surface receptor SORT1. The Sortilin receptor is expressed in a high percentage of tumor tissue from triple-negative breast cancer patients and, as such, represents a novel target for this challenging sub-type of breast cancer.
Peptide engagement of SORT1 efficiently internalizes the peptide-receptor complex which is subsequently targeted to the endosomal compartment for cargo release. These SORT1 engaging peptides serve as a platform for cargo delivery via conjugation of the peptides to therapeutic modalities including cytotoxic agents, oligonucleotides, and radioisotopes.
PQ203
PQ203 for Advanced Solid Tumors including Breast Cancer
PQ203, our most advanced AI-designed peptide-drug conjugate, is currently in a Phase 1 clinical trial in patients with advanced solid tumors, including those with triple-negative breast cancer. PQ203 is composed of a Sortilin-targeting peptide that is conjugated to the cytotoxic agent MMAE. This lead program combines a potent payload with unique pharmacokinetics and an ideal clearance profile suited to that payload.
Our lead asset has exhibited robust efficacy in 17+ in vivo models and much of the data was shared at AACR 2024 and SABCS 2024 (click to view our posters). PQ203 is currently being assessed for safety, tolerability and initial signals of tumor inhibition in a Phase 1A/B clinical trial in Canada and the United States.
ProteinQure Expanded Use Statement
ProteinQure is committed to establishing the safety, tolerability, and efficacy of PQ203 through well-designed clinical studies conducted under the regulations and oversight of health authorities in regions such as Canada, the United States, and Europe. Data generated from these studies are expected to serve as the foundation for future requests for market approval, with the goal of making PQ203 available to patients who need it or may benefit from it.
At this time, ProteinQure is not providing Expanded Access to PQ203. Expanded Access will only be considered once sufficient safety and efficacy information has been obtained through clinical trials. Until then, participation in clinical studies remains the only pathway to access PQ203.
Collaborating
Partner With Us
We are also exploring a breadth of additional opportunities to design peptide-based delivery therapeutics to target cell surface receptors with tissue and cell type specificity. We are open to working with collaborators interested in delivery to tissues where we have already identified receptors amenable to our discovery process.
Our current focus includes the central nervous system, the kidney (proximal tubules and podocytes) and blood brain barrier transport.

